Researchers Crack Marine Methane Paradox
Marine chemists have faced an elusive paradox for decades. The surface waters of the world’s oceans are supersaturated with the greenhouse gas methane, yet most species of microbes that can generate the gas can’t survive in oxygen-rich surface waters. So where exactly does all the methane come from? This longstanding riddle, known as the “marine methane paradox,” may have finally been cracked thanks to a new study by researchers from the Woods Hole Oceanographic Institution (WHOI) and University of Hawai‘i at Mānoa.
According to the study, published in November 2016 in the journal “Nature Geoscience,” the answer may lie in the complex ways that bacteria break down dissolved organic matter, a cocktail of substances excreted into seawater by living organisms.
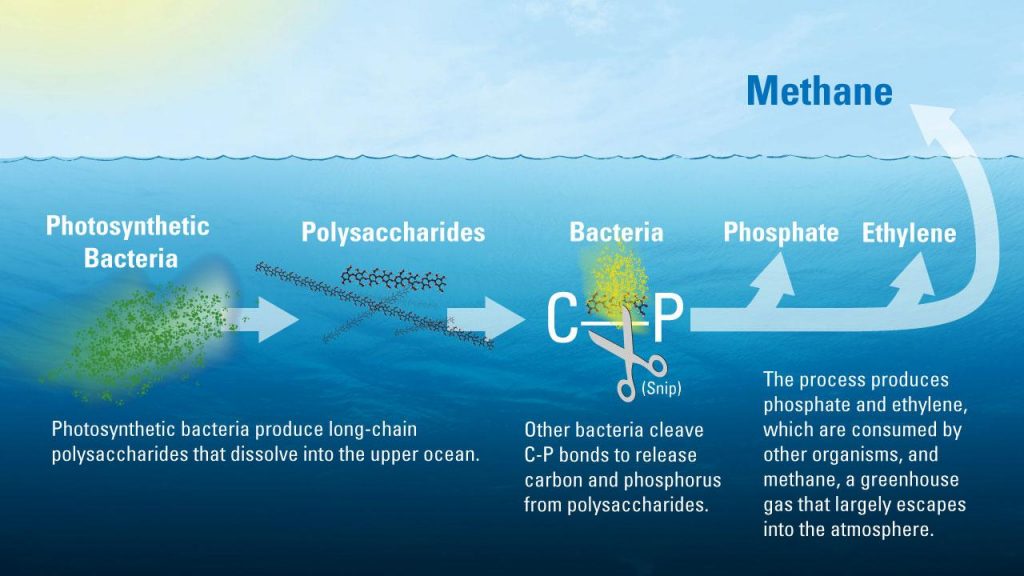
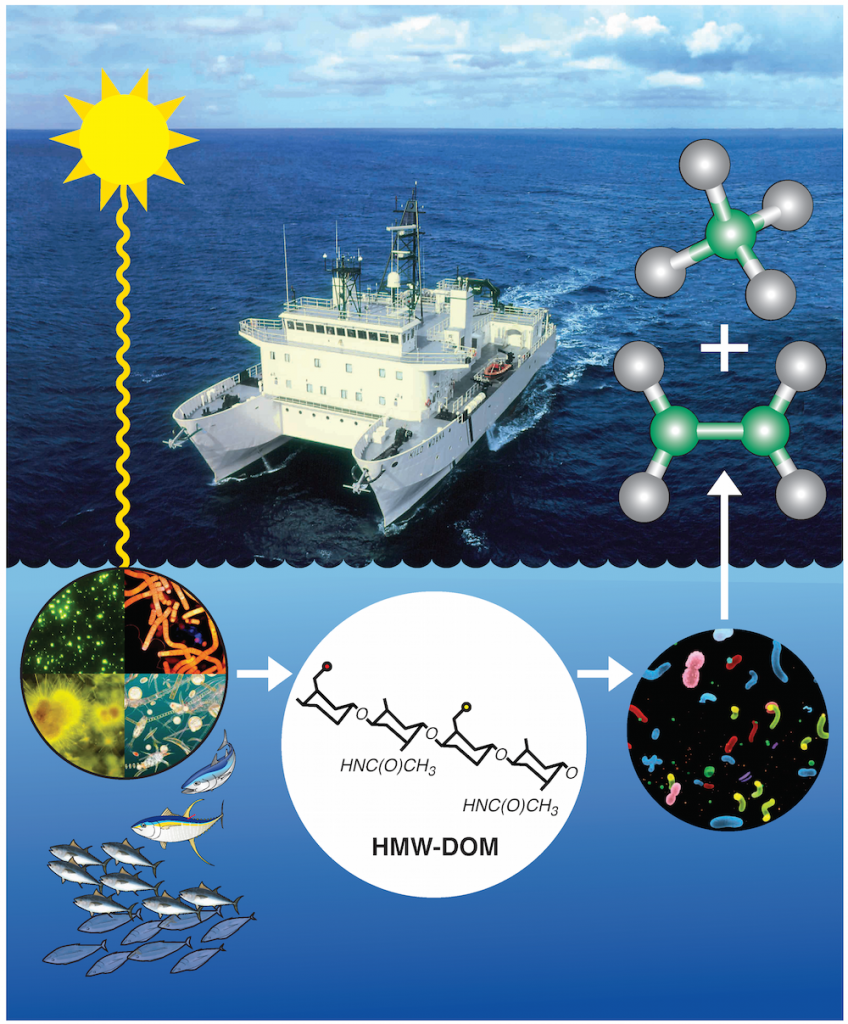
The researchers found that much of the ocean’s dissolved organic matter is made up of novel polysaccharides—long chains of sugar molecules created by photosynthetic bacteria in the upper ocean. Bacteria begin to slowly break these polysaccharides, tearing out pairs of carbon and phosphorus atoms (called C-P bonds) from their molecular structure. In the process, the microbes create methane, ethylene and propylene gasses as byproducts. Most of the methane escapes back into the atmosphere.
“All the pieces of this puzzle were there, but they were in different parts, with different people, in different labs, at different times,” said WHOI geochemist and lead author Dan Repeta. “This paper unifies a lot of those observations.”
Methane is a potent greenhouse gas, and it is important to understand the various sources of methane in the atmosphere. The research team’s findings describe a totally new pathway for the microbial production of methane in the environment, that is very unlike all other known pathways.
Prior to this study, scientists had long suspected that microbes were involved in creating methane in the ocean, but were unable to identify the exact ones responsible. Researchers investigated microbes living in isolated low-oxygen environments, like the guts of fish or shrimp, or flocculent material—snowy-looking bits of animal excrement and other organic material floating in ocean waters. Ultimately, none of those turned out to be a major methane source.
In 2009, David Karl, Ph.D. Victor, and Peggy Brandstrom Pavel, professor of oceanography at UHM and co-author of the recent study, found an important clue to the puzzle. In the lab, he added a manmade chemical called methylphosphonate, which is rich in C-P bonds, to samples of seawater. As he did, bacteria within the samples immediately started making methane, proving that they were able to take advantage of the C-P bonds provided by the chemical. Since methylphosphonate had never been detected in the ocean, the research team reasoned that bacteria in the wild must be finding another natural source of C-P bonds. Exactly what that source was, however, remained elusive.
After analyzing samples of dissolved organic matter from surface waters in the northern Pacific, they ran into a possible solution. The polysaccharides within it turned out to have C-P bonds identical to the ones found in methylphosphonate—and if bacteria could break down those molecules, they might be able to access the phosphorus contained within it.
To confirm this idea, the researchers incubated seawater bacteria under different conditions, adding nutrients such as glucose and nitrate to each batch. Nothing seemed to help the bacteria produce methane—until, that is, they added pure polysaccharides isolated from seawater. Once those were in the mix, the bacteria’s activity spiked, and the vials began spitting out large amounts of methane.
“That made us think it’s a two-part system,” said Repeta. ” You have one species that makes C-P bonds but can’t use them, and another species that can use them but not make them.”
Edward DeLong and Oscar Sosa, UHM microbial oceanographers and co-authors, then began to explore how bacteria metabolize dissolved organic matter. Using a process called metagenomics, they cataloged all the genes he could find in a sample of seawater from the North Pacific. In the process, they found genes responsible for breaking apart C-P bonds, which would allow bacteria to wrench phosphorus away from carbon atoms. Although DeLong was not certain which bacteria could actually do this, one thing was clear: If the gene was active, it would give an organism access to an important but rare nutrient in seawater.
Although the latest paper confirms that it is indeed possible for bacteria to break apart C-P bonds, the authors note that it’s still not a particularly easy means of getting nutrients. With phosphorus tied up in organic molecules, it can be exceedingly difficult for bacteria to reach. If microbes can find other sources of the nutrient, he says, they will inevitably use those first.
“Think of it like a buffet,” Repeta said. “If you’re a microbe, inorganic nutrients are like fruits and meats and all the tasty stuff that you reach for immediately. Organic nutrients are more like leftover liver. You don’t really want to eat it, but if you’re hungry enough, you will. It takes years for bacteria to get around to eating the organic phosphorus in the upper ocean. We don’t exactly know why, but there’s another really interesting story there if we can figure it out.”
“This study proves the value of collaboration in science,” said Karl. “No single person of our research group could have solved this important puzzle alone.”
Also collaborating on the study were Sara Ferrón and Oscar Sosa from the University of Hawai‘i, Carl Johnson and Marianne Acker of the Woods Hole Oceanographic Institution, and Lucas Repeta of the University of California, Los Angeles.
The research was supported by the Gordon and Betty Moore Foundation, the Simons Foundation and the National Science Foundation.